Hypertension as a Cardiovascular Toxicity of Bruton’s Tyrosine Kinase Inhibitors for Chronic Lymphocytic Leukemia
DOI:
https://doi.org/10.58931/cht.2025.4s0368Abstract
In 2014, ibrutinib, a covalent Bruton’s tyrosine kinase inhibitor (BTKi), became available as a treatment for chronic lymphocytic leukemia (CLL) in Canada. It was welcomed with enthusiasm given its oral administration, lower rate of neutropenia and infections, and efficacy in heavily pretreated and high-risk del17p subtypes, at a time when only chemotherapy and monoclonal antibodies were available. Soon adverse events (AE) due to off-target effects emerged; particularly concerning were cardiac arrhythmias, bleeding, and hypertension (HTN). Second-generation BTKis were designed to target BTK more directly, and fortunately, clinical trials reported a lower rate of cardiac AEs. Less attention has been given to HTN despite it being an important modifiable risk factor for subsequent cardiac AEs. This is particularly relevant for CLL, given that the predominantly elderly patient population with CLL tend to survive as long as age-matched peers. This review focuses on HTN as an adverse effect of BTKis, and recommending a management approach.
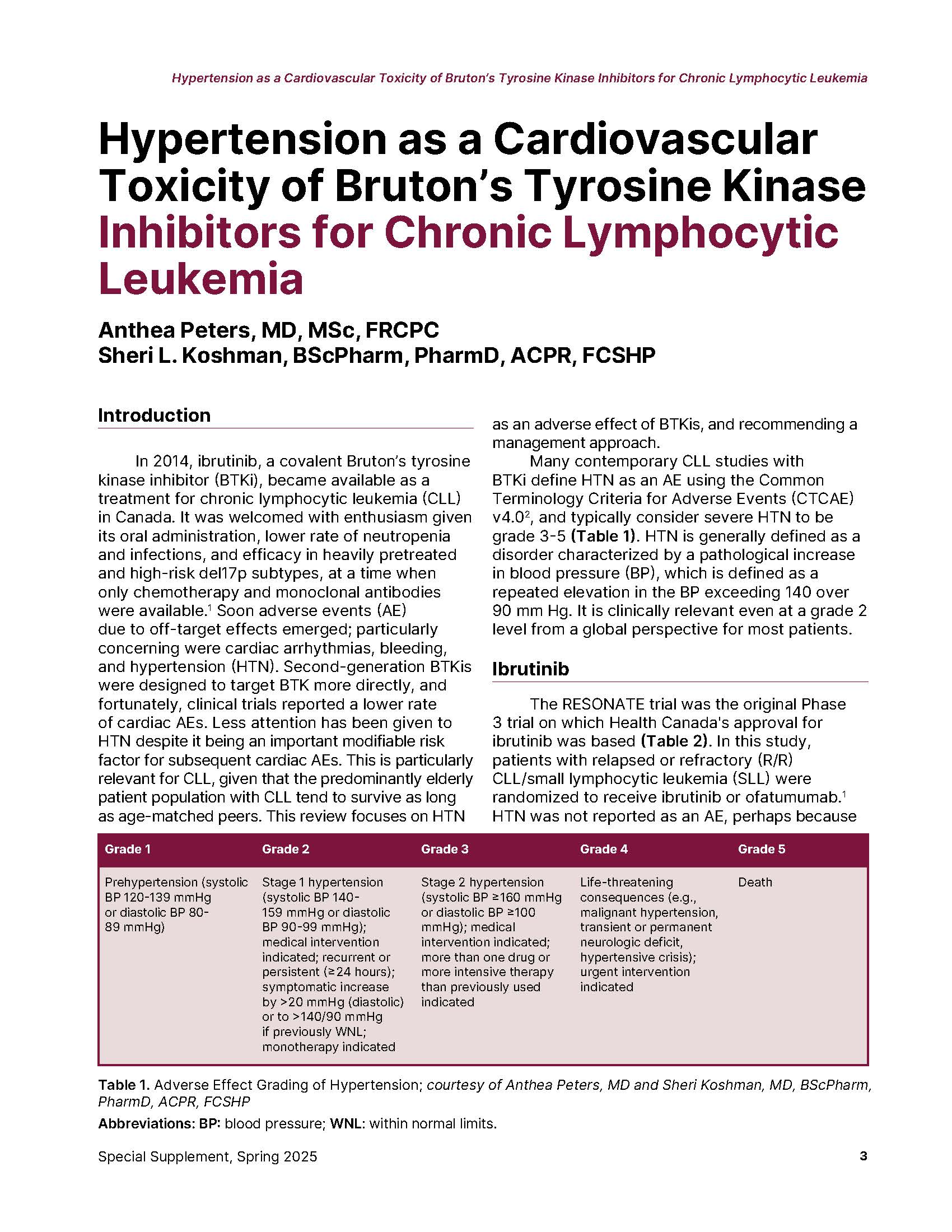
Many contemporary CLL studies with BTKi define HTN as an AE using the Common Terminology Criteria for Adverse Events (CTCAE) v4.0, and typically consider severe HTN to be grade 3-5 (Table 1). HTN is generally defined as a disorder characterized by a pathological increase in blood pressure (BP), which is defined as a repeated elevation in the BP exceeding 140 over 90 mm Hg. It is clinically relevant even at a grade 2 level from a global perspective for most patients.
References
Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371(3): 213-23. DOI: https://doi.org/10.1056/NEJMoa1400376
https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference _8.5x11.pdf.
Byrd JC, Hillmen P, O'Brien S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood 2019; 133(19): 2031-42. DOI: https://doi.org/10.1182/blood-2018-08-870238
Munir T, Brown JR, O'Brien S, et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol 2019; 94(12): 1353-63. DOI: https://doi.org/10.1002/ajh.25638
Barr PM, Owen C, Robak T, et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv 2022; 6(11): 3440-50. DOI: https://doi.org/10.1182/bloodadvances.2021006434
Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020; 34(3): 787-98. DOI: https://doi.org/10.1038/s41375-019-0602-x
Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N Engl J Med 2018; 379(26): 2517-28. DOI: https://doi.org/10.1056/NEJMoa1812836
Woyach JA, Perez Burbano G, Ruppert AS, et al. Follow-up from the A041202 study shows continued efficacy of ibrutinib regimens for older adults with CLL. Blood 2024; 143(16): 1616-27. DOI: https://doi.org/10.1182/blood.2023021959
Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N Engl J Med 2019; 381(5): 432-43. DOI: https://doi.org/10.1056/NEJMoa1817073
Shanafelt TD, Wang XV, Hanson CA, et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood 2022; 140(2): 112-20. DOI: https://doi.org/10.1182/blood.2021014960
Hillmen P, Pitchford A, Bloor A, et al. Ibrutinib and rituximab versus fludarabine, cyclophosphamide, and rituximab for patients with previously untreated chronic lymphocytic leukaemia (FLAIR): interim analysis of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2023; 24(5): 535-52. DOI: https://doi.org/10.1016/S1470-2045(23)00144-4
Kater AP, Owen C, Moreno C, et al. Fixed-Duration Ibrutinib-Venetoclax in Patients with Chronic Lymphocytic Leukemia and Comorbidities. NEJM Evid 2022; 1(7): EVIDoa2200006. DOI: https://doi.org/10.1056/EVIDoa2200006
Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS One 2019; 14(2): e0211228. DOI: https://doi.org/10.1371/journal.pone.0211228
Gordon MJ, Jones JE, George B, et al. Long-term outcomes in patients with chronic lymphocytic leukemia treated with ibrutinib: Focus on hypertension and cardiovascular toxicity. Cancer 2023; 129(14): 2192-200. DOI: https://doi.org/10.1002/cncr.34787
Dickerson T, Wiczer T, Waller A, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 2019; 134(22): 1919-28. DOI: https://doi.org/10.1182/blood.2019000840
Ghia P, Pluta A, Wach M, et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J Clin Oncol 2020; 38(25): 2849-61. DOI: https://doi.org/10.1200/JCO.19.03355
Ghia P, Pluta A, Wach M, et al. Acalabrutinib Versus Investigator's Choice in Relapsed/Refractory Chronic Lymphocytic Leukemia: Final ASCEND Trial Results. Hemasphere 2022; 6(12): e801. DOI: https://doi.org/10.1097/HS9.0000000000000801
Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet 2020; 395(10232): 1278-91. DOI: https://doi.org/10.1016/S0140-6736(20)30262-2
Sharman JP, Egyed M, Jurczak W, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naive chronic lymphocytic leukemia. Leukemia 2022; 36(4): 1171-5. DOI: https://doi.org/10.1038/s41375-021-01485-x
Brown JR, Seymour JF, Jurczak W, et al. Fixed-Duration Acalabrutinib Combinations in Untreated Chronic Lymphocytic Leukemia. N Engl J Med 2025; 392(8): 748-62. DOI: https://doi.org/10.1056/NEJMoa2409804
Brown JR, Byrd JC, Ghia P, et al. Cardiovascular adverse events in patients with chronic lymphocytic leukemia receiving acalabrutinib monotherapy: pooled analysis of 762 patients. Haematologica 2022; 107(6): 1335-46. DOI: https://doi.org/10.3324/haematol.2021.278901
Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J Clin Oncol 2021; 39(31): 3441-52. DOI: https://doi.org/10.1200/JCO.21.01210
Seymour JF, Byrd JC, Ghia P, et al. Detailed safety profile of acalabrutinib vs ibrutinib in previously treated chronic lymphocytic leukemia in the ELEVATE-RR trial. Blood 2023; 142(8): 687-99. DOI: https://doi.org/10.1182/blood.2022018818
Chen ST, Azali L, Rosen L, et al. Hypertension and incident cardiovascular events after next-generation BTKi therapy initiation. J Hematol Oncol 2022; 15(1): 92. DOI: https://doi.org/10.1186/s13045-022-01302-7
Brown JR, Eichhorst B, Hillmen P, et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med 2023; 388(4): 319-32. DOI: https://doi.org/10.1056/NEJMoa2211582
Shadman M, Munir T, Robak T, et al. Zanubrutinib Versus Bendamustine and Rituximab in Patients With Treatment-Naive Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: Median 5-Year Follow-Up of SEQUOIA. J Clin Oncol 2024: JCO2402265. DOI: https://doi.org/10.1182/blood-2024-194864
Brown JR, Ghia P, Jurczak W, et al. Characterization of zanubrutinib safety and tolerability profile and comparison with ibrutinib safety profile in patients with B-cell malignancies: post-hoc analysis of a large clinical trial safety database. Haematologica 2024; 109(7): 2277-83. DOI: https://doi.org/10.3324/haematol.2023.283846
Moslehi JJ, Furman RR, Tam CS, et al. Cardiovascular events reported in patients with B-cell malignancies treated with zanubrutinib. Blood Adv 2024; 8(10): 2478-90. DOI: https://doi.org/10.1182/bloodadvances.2023011641
Kittai AS, Allan JN, James D, et al. An indirect comparison of acalabrutinib with and without obinutuzumab vs zanubrutinib in treatment-naive CLL. Blood Adv 2024; 8(11): 2861-9. DOI: https://doi.org/10.1182/bloodadvances.2023012142
Yin S, Zheng X, Zhang W, et al. Efficacy and safety of new-generation Bruton tyrosine kinase inhibitors in chronic lymphocytic leukemia/small lymphocytic lymphoma: a systematic review and meta-analysis. Ann Hematol 2024; 103(7): 2231-44. DOI: https://doi.org/10.1007/s00277-023-05486-x
Lyon AR, Lopez-Fernandez T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J Cardiovasc Imaging 2022; 23(10): e333-e465.
Rabi DM, McBrien KA, Sapir-Pichhadze R, et al. Hypertension Canada's 2020 Comprehensive Guidelines for the Prevention, Diagnosis, Risk Assessment, and Treatment of Hypertension in Adults and Children. Can J Cardiol 2020; 36(5): 596-624. DOI: https://doi.org/10.1016/j.cjca.2020.02.086
Soumerai JD, Barrientos JC, Ahn IE, et al. Consensus Recommendations from the 2024 Lymphoma Research Foundation Workshop on Treatment Selection and Sequencing in CLL or SLL. Blood Adv 2024. DOI: https://doi.org/10.1182/bloodadvances.2024014474
Quartermaine C, Ghazi SM, Yasin A, et al. Cardiovascular Toxicities of BTK Inhibitors in Chronic Lymphocytic Leukemia: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2023; 5(5): 570-90. DOI: https://doi.org/10.1016/j.jaccao.2023.09.002
Awan FT, Addison D, Alfraih F, et al. International consensus statement on the management of cardiovascular risk of Bruton's tyrosine kinase inhibitors in CLL. Blood Adv 2022; 6(18): 5516-25. DOI: https://doi.org/10.1182/bloodadvances.2022007938
Samples L, Voutsinas J, Fakhri B, et al. Hypertension treatment for patients receiving ibrutinib: a multicenter retrospective study. Blood Adv 2024; 8(9): 2085-93. DOI: https://doi.org/10.1182/bloodadvances.2023011569
Larsson K, Mattsson M, Ebrahim F, Glimelius I, Hoglund M. High prevalence and incidence of cardiovascular disease in chronic lymphocytic leukaemia: a nationwide population-based study. Br J Haematol 2020; 190(4): e245-e8. DOI: https://doi.org/10.1111/bjh.16859
Wang Y, Achenbach SJ, Rabe KG, et al. Cause of death in patients with newly diagnosed chronic lymphocytic leukemia (CLL) stratified by the CLL-International Prognostic Index. Blood Cancer J 2021; 11(8): 140. DOI: https://doi.org/10.1038/s41408-021-00532-1
Mancia G, Facchetti R, Quarti-Trevano F, Grassi G. Reproducibility and Treatment Effect on Office and Ambulatory Pressure Relation. Hypertension 2025; 82(1): 126-35. DOI: https://doi.org/10.1161/HYPERTENSIONAHA.124.23549
Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med 2015; 373(25): 2425-37. DOI: https://doi.org/10.1056/NEJMoa1509388
Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20(1): 43-56. DOI: https://doi.org/10.1016/S1470-2045(18)30788-5
Brown JR, Eichhorst B, Lamanna N, et al. Sustained benefit of zanubrutinib vs ibrutinib in patients with R/R CLL/SLL: final comparative analysis of ALPINE. Blood 2024; 144(26): 2706-17. DOI: https://doi.org/10.1182/blood.2024024667
Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol 2022; 23(8): 1031-43. DOI: https://doi.org/10.1016/S1470-2045(22)00293-5
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Canadian Hematology Today

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
